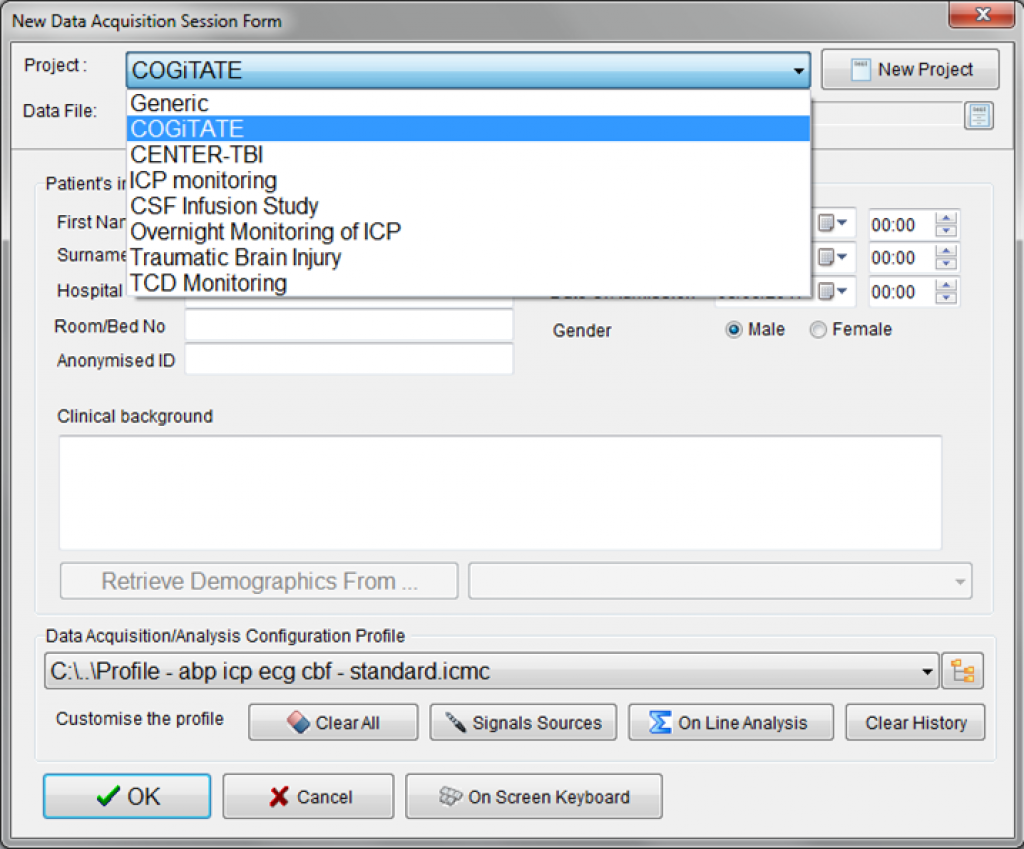
ICM+ was found to be versatile and robust enough to be employed as the main data collection tool in multi-centre clinical trials. With the new ICM+ Clinical Trial Assistant, it is possible to configure the software to aid in the execution of clinical trials.

CENTER-TBI is a large European project that aims to improve care for patients with Traumatic Brain Injury (TBI). In this project, ICM+ was the cornerstone of the high-resolution sub-study where it served as the main data collection system used in 20 centres all over Europe.
During the study, 250+ patients were recruited for high-resolution monitoring with a total recording time of more than 1,700 days.
Despite the innate differences throughout all the participating centres, be it in different work methods and monitoring devices, ICM+ was capable of successfully gathering all the data in a robust and homogeneous way.

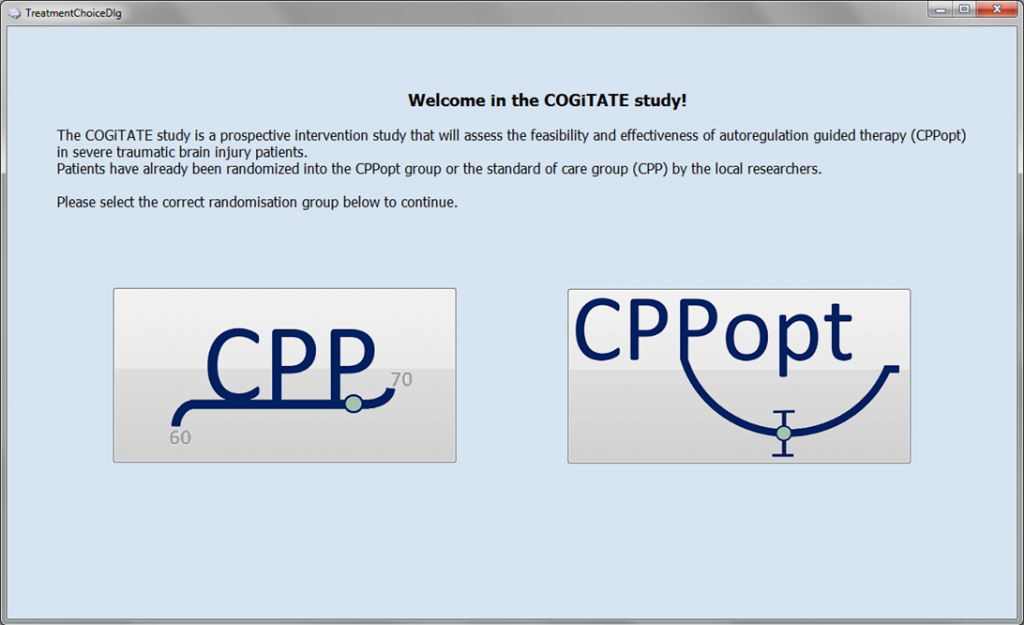
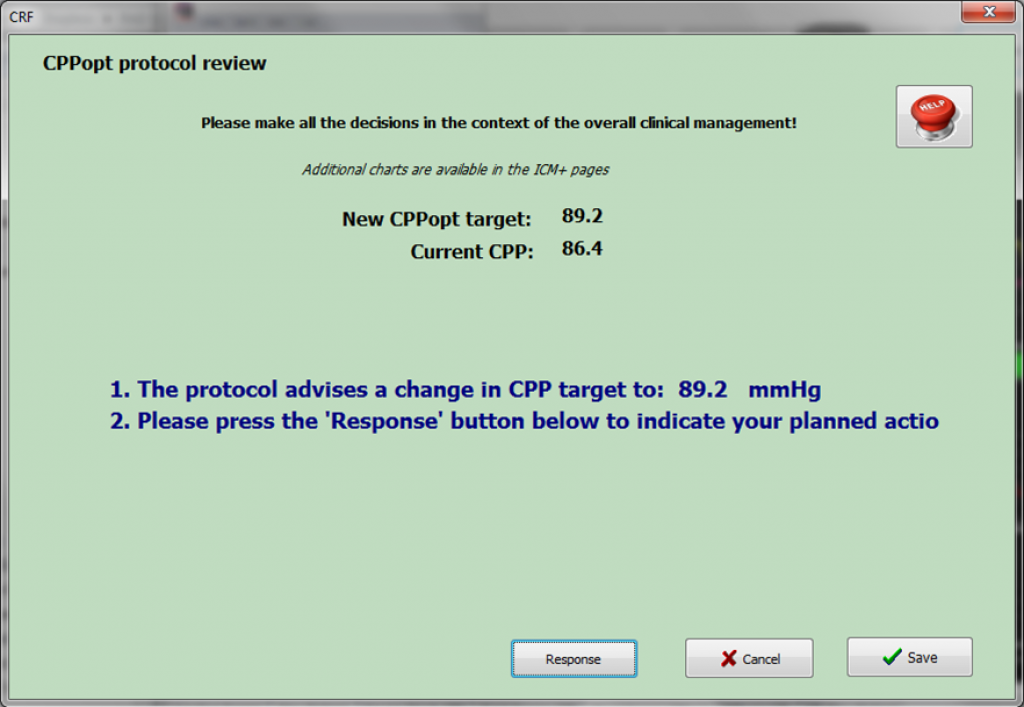
This is a feasibility study that aims to test the use of the CPPopt-guided therapy prospectively in clinical practice (trial link). The study will be conducted in 3 centres: Cambridge, Leuven, and Maastricht.
This will be the first time that the new Clinical Trial Assistant will be put to use.